Contact us
Engineering Immunity: The Science Behind CAR T-Cell Customization
By Shaivana Bano
21 September 2025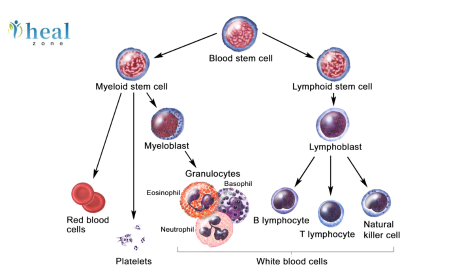
- Origins and Evolution of CAR T-Cell Therapy
- Anatomy of a CAR: Design Principles
- Generations of CAR design have evolved:
- Gene-Editing Tools for CAR T-Cell Engineering
- Strategies for Customising CAR T Cells
- In Which Diseases is it used?
- What are the Challenges in CAR T cell therapy?
- What is The Future of CAR T cell therapy?
- Conclusion
In recent years, immunotherapy has revolutionised the landscape of cancer treatment, with Chimeric Antigen Receptor (CAR) T-cell therapy standing out as one of the most promising approaches. By reprogramming a patient’s own immune cells to recognise and eliminate malignant cells, CAR T-cell therapy bridges the gap between biotechnology and clinical oncology. At the heart of its success lies a profound mastery of gene-editing and cellular engineering, enabling scientists to tailor immune responses with remarkable precision. This blog explores the science behind CAR T-cell customisation, covering its history, design strategies, gene-editing tools, therapeutic applications, challenges, and the future of engineered immunity.
Origins and Evolution of CAR T-Cell Therapy
The concept of harnessing immune cells to fight cancer dates back decades. Early research in the 1980s laid the groundwork for the genetic modification of T cells. By the 1990s, scientists designed the first CARs, which combined the antigen-recognising domain of an antibody with T-cell signalling molecules. While these early iterations lacked efficacy and durability, iterative improvements over the past 20 years have transformed CAR T-cell therapy into a viable clinical option.
The FDA approval of tisagenlecleucel in 2017 for pediatric acute lymphoblastic leukaemia marked a historic milestone. Since then, multiple CAR T-cell therapies have been approved, targeting blood cancers such as lymphoma and multiple myeloma, with ongoing research to expand their reach to solid tumours.
Anatomy of a CAR: Design Principles
A CAR is an engineered receptor that redirects T-cell specificity toward tumour-associated antigens. Its structure consists of four major components:
- Extracellular Antigen-Binding Domain: Typically derived from a single-chain variable fragment (scFv) of an antibody, this domain dictates the CAR’s target specificity.
- Hinge and Transmembrane Domains: These structural elements provide flexibility and stability, anchoring the receptor within the T-cell membrane.
- Intracellular Signalling Domain: The CD3ζ chain initiates T-cell activation upon antigen engagement.
- Costimulatory Domains: Additional domains (such as CD28 or 4-1BB) enhance persistence, proliferation, and anti-tumour activity.
Generations of CAR design have evolved:
- First-generation CARs: Contained only the CD3ζ signalling domain.
- Second-generation CARs: Added one costimulatory domain (CD28 or 4-1BB).
- Third-generation CARs: Integrated multiple costimulatory domains.
- Fourth-generation CARs (TRUCKs): Include inducible cytokine expression to enhance anti-tumour responses further.
Gene-Editing Tools for CAR T-Cell Engineering
Gene-editing is central to customising CAR T cells. Traditional viral vectors (retroviruses, lentiviruses) remain widely used for stable CAR integration. However, new gene-editing technologies have expanded the possibilities:
- CRISPR-Cas9: Enables precise insertion or knockout of genes, allowing for removal of inhibitory receptors (e.g., PD-1) or targeted integration of CAR constructs.
- TALENs and Zinc-Finger Nucleases (ZFNs): Earlier genome editing tools are still relevant for clinical applications.
- Transposon Systems (Sleeping Beauty, PiggyBac): Non-viral alternatives for safer, cost-effective CAR delivery.
- Base Editing and Prime Editing: Emerging technologies for fine-tuned genetic modifications without double-strand breaks.
These tools empower scientists to optimise CAR T-cell persistence, safety, and efficacy.
Strategies for Customising CAR T Cells
Customisation of CAR T cells extends beyond receptor design. Researchers are developing multi-layered engineering strategies:
- Target Antigen Selection: Choosing tumour-specific or overexpressed antigens (CD19, BCMA, HER2) to maximise efficacy and minimise off-tumour toxicity.
- Dual or Tandem CARs: Equipping T cells with receptors for two antigens to prevent antigen escape by tumours.
- Armoured CARs: Engineering T cells to secrete cytokines (IL-12, IL-18) that modulate the tumour microenvironment.
- Safety Switches: Incorporating suicide genes or small-molecule-controlled switches to halt CAR T activity in case of severe toxicity.
- Allogeneic CAR T Cells: Using gene-editing to create universal, off-the-shelf CAR T cells from healthy donors, eliminating the need for patient-specific manufacturing.
- Resistance to Tumour Suppression: Deleting checkpoint molecules (PD-1, CTLA-4) or overexpressing costimulatory ligands to counteract immunosuppressive signals.
- Metabolic Reprogramming: Enhancing mitochondrial fitness and energy utilisation to sustain CAR T activity in nutrient-deprived tumour environments.
In Which Diseases is it used?
CAR T-cell therapy has demonstrated exceptional success in haematological malignancies. Approved therapies target:
- CD19: Used in:
- B-cell malignancies
- Acute lymphoblastic leukaemia (ALL)
- Non-Hodgkin lymphoma (NHL)
- Chronic lymphocytic leukaemia (CLL).
- BCMA: Effective in multiple myeloma.
Research is expanding into solid tumours, although challenges such as heterogeneous antigen expression, poor T-cell trafficking, and immunosuppressive tumour microenvironments persist. Novel strategies include:
- Locally delivered CAR T cells to tumour sites.
- Incorporating chemokine receptors to improve trafficking.
- Engineering CAR T cells to resist exhaustion.
Beyond oncology, CAR T cells are being investigated for:
- Autoimmune diseases (such as lupus and rheumatoid arthritis)
- Infectious diseases (HIV, hepatitis B)
- Fibrotic conditions (including cardiac and liver fibrosis)
What are the Challenges in CAR T cell therapy?
Despite its promise, CAR T-cell therapy faces several hurdles:
- Cytokine Release Syndrome (CRS): Overactivation leading to systemic inflammation.
- Neurotoxicity: The mechanisms underlying this condition remain unclear, but it can lead to serious complications.
- On-Target, Off-Tumour Toxicity: Risk when target antigens are also expressed on healthy tissues.
- Manufacturing Limitations: Personalised therapy requires complex, time-consuming production.
- Cost and Accessibility: High costs limit global availability.
Ongoing research aims to mitigate these risks through the development of improved safety switches, universal donor cells, and scalable manufacturing platforms.
What is The Future of CAR T cell therapy?
The future of CAR T-cell therapy lies in its convergence with cutting-edge fields:
- Synthetic Biology: Designing programmable receptors, logic-gated CARs, and synthetic circuits for precise control.
- Artificial Intelligence (AI): Predicting antigen targets, optimising CAR design, and personalising therapy protocols.
- Next-Generation Gene Editing: Refined tools, such as base and prime editing, promise safer and more efficient engineering.
- Off-the-Shelf Therapies: Universal CAR T cells and CAR NK cells for mass production.
- Combination Therapies: Integrating CAR T cells with checkpoint inhibitors, vaccines, or oncolytic viruses.
Ultimately, CAR T-cell therapy may expand from a niche option for refractory cancers to a frontline treatment and beyond, reshaping not only oncology but broader medicine.
Conclusion
CAR T-cell therapy represents a paradigm shift in the treatment of disease. By merging immunology, genetics, and bioengineering, scientists are pushing the boundaries of personalised medicine. From the first-generation receptors to the futuristic concept of programmable synthetic immune cells, CAR T-cell customisation underscores the immense potential of engineered immunity. As we refine gene-editing tools and enhance design strategies, CAR T therapy is poised to become safer, more accessible, and more versatile, offering hope for millions of patients worldwide.
Contact us
Written by
B.Sc. & M.Sc. in Medical Imaging Technology

Dr. Rahul Bhargava
MBBS, MD, DM
Bone Marrow Transplant Specialist, Hemato-Oncologist, Hematologist
22 Years Years of Experience

Latest Blogs
Reviews
I am Fadel Abu Muhammad from Tal Afar, Iraq My father had a brain tumor. We traveled to India to Accord Specialty Hospital, New Delhi, through a resident professor, Muhammad, Dr. Vikram, who called for a laparoscopic tumor removal operation. Thank God I thank the translator, the doctor, and all the hospital staff.
Posted On
I am Muhammad Reda from Baghdad, Iraq I was suffering from weakness in my left hand. I underwent tests and it turned out that I had a brain tumor. The translator, Muqeem Muhammad, contacted us and we traveled to India. I had a neuronavigation procedure performed by Dr. Sandeep Vashya Praise be to God, the operation was completed successfully on the computer At Fortis Hospital New Delhi I thank the translator and the doctor
Posted On
All our gratitude and appreciation to the wonderful translator Mohammed Muqeem, who was truly a great support and companion during our journey for my mother’s treatment in India. He was extremely helpful, deeply understanding of all our needs, patient, and dedicated in his work, which eased a lot of the hardship and challenges we faced being away from home. His presence with us was not just about translating words but about offering real human support in every situation and every moment. May God bless him and reward him greatly for all he has done for us.
Posted On
I'm Emad Mohammed Khadir from Iraq. I was suffering from cirrhosis and liver cancer. I came to Fortis Hospital in India to see Dr. Ankur Bahil, a consultant oncologist, with the help of our translator, resident professor Mohammed Al Hindi. Thank God, we received our chemotherapy doses. The doctor and all the hospital staff were very kind and helpful. May God bless them all. Thank you.
Posted On
I am Redha Fadel from Nasiriyah, Iraq. I had a bone tumor, osteosarcoma. I traveled to India to see Dr. Ankur, a cancer specialist at Fortis Hospital, with the help of Hill Zone Medical Tourism Company, Professor Muqeem Muhammad Al Hindi. I thank you for your humanity and services.
Posted On
Peace be upon you. I am from Iraq. My brother is suffering from a tumor in his left thigh (osteosarcoma). We came to Al-Nahda for treatment. We stayed at Fortis Hospital for two months. We are now going to Iraq and will return to India to complete the treatment and the joint replacement surgery. I would like to thank the polite Indian translator, Muqeem Mohammed Al-Hindi. He was our brother before he was a translator and he helped us a lot with my brother's illness. Thank you, Muqeem. Thank you, Fortis Hospital. Thank you, Dr. Ankur.
Posted On
Peace be upon you, I'm from Iraq. My name is Majid Mazhar Kazim. My brother was suffering from tumors, so we contacted a resident professor, Muhammad Al-Hindi, and we sent him medical reports. He received us from the airport in New Delhi. I highly recommend him to Iraqi patients. He is helpful and well-mannered. He didn't hold back on us and didn't leave us alone from the first day until the last. He also took us to the airport. I thank them all.
Posted On
I am Mohammed Kazim from Iraq, from Babylon. My brother Mushtaq was suffering from meningitis. We came to India to Fortis Hospital through our resident professor, Mohammed, the director of Hill Zone Medical Services. I thank them for their kind treatment and translation. It was an enjoyable and fruitful trip. Praise be to God.
Posted On
I am from iraq tilafar, i came to India for my treatment an immune disease, i am thankful to Mr Muqeem and hospital staff, Marego Asia Hospital
Posted On
I am from Iraq. My son was suffering from Hirayama disease. I called Heal Zone, and they arranged our treatment journey. Now my son is in good health, and his finger movement has come back.
Posted On